







Findings
Plain films (Figure 1 and Figure 2) show “tram track” calcification in the right frontal region. The gyral or subcortical calcification is better seen in the CT image (Figure 3).
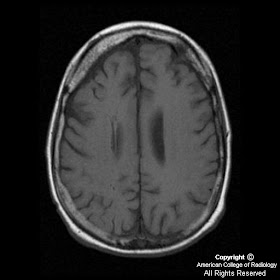
Axial T1 image (Figure 4) demonstrates volume loss in the right frontal area with thickened diploic space in this region. Sagittal SE image (Figure 5) shows multiple round signal voids, which are dilated deep medullary veins.
Postcontrast images (Figure 7 and Figure 8) show leptomeningeal enhancement, suggestive of increased venous collaterals and prominence of the right transmantle (transcerebral) veins (Figure 6). Also note the enlarged ipsilateral right choroid plexus (Figure 7).
GRE (Figure 7) shows “blooming” in the right frontal area corresponding to the area of calcification seen on CT.
MRV (Figure 9) findings demonstrate a hypoplastic right transverse sinus as well as a very small right-sided jugular vein.
Diagnosis: Sturge-Weber syndrome (SWS)
SWS, also known as encephalotrigeminal angiomatosis or meningiofacial angiomatosis, is a rare neurocutaneous syndrome that includes a facial port wine stain and associated leptomeningeal angiomatosis. The pial angiomatosis may be bilateral in 20% of cases.
It is generally considered nonhereditary. Embryologically, the abnormality most likely develops at gestational weeks four to eight. It is hypothesized that impaired venous outflow, caused by loss of normal connections between cortical veins and dural and calvarial circulation, results in persistent, primitive vascular plexus. This results in poor venous drainage from the cerebral cortex and progressive cortical damage.
Seizures often begin in the first year of life. It is the presenting feature in 80% of patients and may be generalized or partial. Hemiparesis may occur in up to 30% of cases resulting from hemiatrophy of brain. Developmental delay is common.
The port wine stain (nevus flammeus) usually occurs in the distribution of first and second division of trigeminal nerve. The choroid of eye may be involved with patients developing glaucoma.
The impaired venous drainage results in progressive ischemia of underlying brain and, eventually, progressive cortical atrophy and calcification. The cortical calcifications are rarely identified at birth. The most common area involved is the parieto-occipital region. Skull radiographs may show the typical “tram track” calcifications in apposing gyri. CT may also show enlargement of adjacent diploic space, hyperpneumatizaton of ipsilateral sinuses, and mastoid air cells.
MRI may show signs of ischemia and gliosis in early stages, but parenchymal atrophy over time. Gadolinium-enhanced MRI is highly sensitive to meningeal enhancement. Calcifications are better detected with gradient echo techniques. Enlargement of the ipsilateral choroid plexus may be secondary to hyperplasia or angiomatous involvement.
MR venogram may show enlarged deep collateral (medullary or subependymal) veins with lack of superficial cortical veins.
PET may show increased metabolism in early stages, which can be helpful in surgical planning.







