 Findings
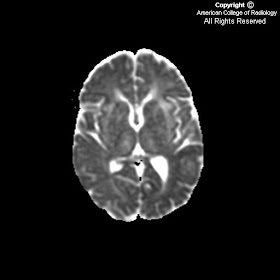
FindingsMRI of the brain with diffusion weighted image (Figure 5), ADC map (Figure 6) and multiple FLAIR images (Figures 1, 2, 3 and 4) show marked abnormal T2 signal hyperintensity in the subcortical and deep white matter of the temporal (Figure 4) and frontal lobes (Figure 1 and Figure 2). None of the areas correlate with restricted diffusion (Figure 5 and Figure 6) or acute infarction. In Figure 3, signal hyperintensity involves the external capsules, basal ganglia and thalami bilaterally. Notably absent is abnormal decreased signal in the corpus callosum on the T1 Sagittal and none of the signal hyperintensity in the periventricular white matter is oriented perpendicularly to the lateral ventricles.
Differential diagnosis:
- CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoenceopalopathy)
- Sporadic subcortical ateriosclerotic encephalopathy (sSAE) – This is similar to CADASIL. CADASIL typically has bilateral anterior temporal and superior frontal lobe involvement which is not as commonly seen in sSAE.
- Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) – As opposed to CADASIL, areas of signal intensity associated with MELAS will frequently disappear with clinical improvement. Onset of clinical symptoms occur as early as 15 years of age and include seizures and acute stroke like symptoms.
- Hypercoagulable states (Antiphospholipid syndrome, protein S deficiency) – Differentiated by abnormal laboratory values in a young patient with multiple recurrent TIA’s/Strokes.
- Vasculitis – differentiated based on laboratory values (elevated sedimentation rate) and angiographic findings. Angiography is normal in patients with CADASIL.
Diagnosis: CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoenceopalopathy)CADASIL, or cerebral autosomal dominant ateriopathy with subcortical infarcts and leukoenceopalopathy, is a rare (1/100,000) hereditary, non-atheromatous vasculopathy found in young adults. Patients suffer from a autosomal dominant mutation in the NOTCH3 gene, which codes for a large transmembrane receptor that is found in abnormally high concentrations on the surface of vascular smooth muscle cells. Pathologically this manifests as a non-arteriosclerotic amyloid-negative angiopathy primarily affecting perforating and meningeal arteries. Histologically, a characteristic granular osmiophilic material is found in the vascular basal membrane.
This leads to two lesion types that have been described:
- 1) Degenerated and destroyed vascular smooth muscle cells leading to impaired vasodilation in response to hypercarbia
- 2) Fibrous thickening of the arteriolar wall leading to arteriolar narrowing and compromising basal cerebral blood flow
Clinically, common presenting signs and symptoms include recurrent TIAs, cognitive deficits, migraine with aura, depression, and rarely seizures. The classic profile is that of a young adult with recurrent TIAs and a history of migraine with aura. There is no gender predilection.
Imaging findings are most characteristic with MR. T2 and FLAIR images show diffuse white matter and lacunar subcortical hyperintensities. The frontal and temporal lobes as well as the insulae are most commonly involved. Anterior temporal poles and external capsule hyperintensities carry higher diagnostic accuracy for CADASIL. The periventricular white matter and cortex are generally spared, but basal ganglia and brainstem can be involved. T1 images show both large coalescent white matter isointense lesions, as well as small well circumscribed subcortical hypointensities. Digital subtraction angiography is normal, and is done to exclude vasculitis and hypercoagulable states.
Genetic testing for NOTCH3 mutations associated with CADASIL are available. The patient had tested positive for this abnormality, and naturally had a family history of this autosomal dominant disorder.