 Findings
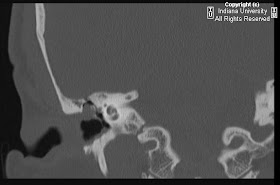
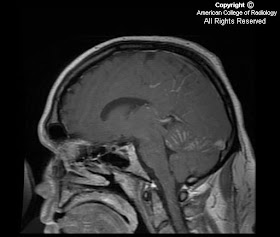
FindingsFigure 1, Figure 2, Figure 3, and Figure 4: Postgadolinium T1 weighted images demonstrate linear contrast enhancement in the subarachnoid space, most notably interdigitating between the folia of the superior cerebellum and in the sulci of the parietal and occipital lobes. The T1 weighted precontrast images are unremarkable.
On FLAIR imaging in Figure 5, there is corresponding hyperintensity in the subarachnoid space.
Chest TC (not shown) demonstrates a high-attenuation central mass within the esophagus, which is expanding the lumen.
Diagnosis: Leptomeningeal carcinomatosis from esophageal adenocarcinoma The leptomeninges consist of two layers; the pia mater and the arachnoid mater, which enclose the subarachnoid space and the CSF. The leptomeninges are a frequently missed site of metastastic involvement, especially for non-hematologic primary malignancies. There are many proposed routes of entry for tumor cells into the CSF including hematogenous spread via the arachnoid vessels or choroid plexus, direct extension from the skull, vertebrae, dura or retrograde perineural spread via the peripheral or cranial nerves. Once tumor cells reach the CSF, rapid dissemination can occur.
Up to 50% of patients with leptomeningeal metastases present with signs of increased intracranial pressure and/or hydrocephalus including headaches, back pain, nausea, vomiting and dizziness. These symptoms are most likely secondary to obstruction of CSF flow by tumor cells. Other clinical manifestations include seizures, focal cranial nerve deficits and meningeal signs such as nuchal rigidity and photophobia.
CSF cytology is the definitive test for diagnosis of leptomeningeal involvement. However, while highly specific, cytology is often falsely negative. The accuracy of a single lumbar puncture is approximately 50%, which increases to 90% with three LPs. Cytology remains negative in 10-20% of patients, presumably in situations in which the malignant cells are more adherent to the leptomeninges.
Contrast enhanced MR imaging is the diagnostic test of choice as an adjunct to CSF cytology when leptomeningeal metastases are suspected. Gadolinium enhanced MRI is more sensitive than a single lumbar puncture, but is less specific. The most common imaging findings include diffuse leptomeningeal contrast enhancement, multiple masses or nodules within the subarachnoid space and/or hydrocephalus. The diffuse leptomeningeal enhancement pattern has been referred to as sugar-coating or zuckerguss (German for icing or sugar-coating). Studies have shown that contrast-enhanced T1-weighted MR imaging is the most sensitive single sequence for detection of leptomeningeal metastases.
Prognosis for leptomeningeal metastases is poor. Without treatment, the average survival is 1-2 months. Treatment options include corticosteroids, intrathecal chemotherapy and radiation therapy. Despite aggressive therapy, the median survival in most randomized-controlled trials is 3-4 months.