Findings
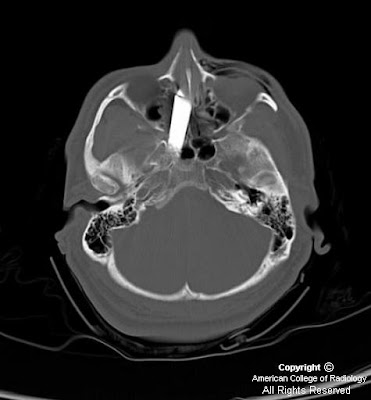
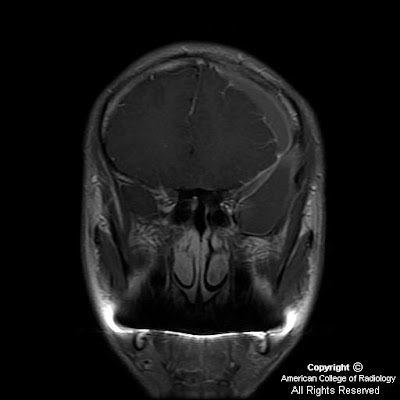
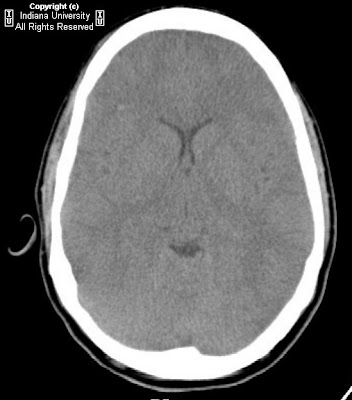
Heterogeneously enhancing mass centered at the junction of the left nasal cavity and ethmoid sinus has eroded through the cribriform plate and extends into the anterior cranial fossa. Sharp interface between the mass and the brain parenchyma, with mass-effect and vasogenic edema in the left frontal lobe. Left orbit medial wall has been eroded, and the extraconal mass has mass-effect on the left medial rectus with the fat plane between the mass and the muscle intact. Mass extends posteriorly to the margin of the left sphenoid sinus Bilateral frontal sinuses are fluid filled with the mass minimally extending into the inferior portion of the right frontal sinus. Ethmoid sinuses are completely occupied by the mass. Right maxillary and sphenoid sinuses are clear. Left maxillary sinus is almost completely fluid filled, and the left osteomeatal unit is completely obstructed by the mass.
Differential diagnosis:
- Esthesioneuroblastoma
- Squamous cell carcinoma
- Sinonasal undifferentiated carcinoma (SNUC)
- Sinonasal melanoma
- Meningioma
- Metastasis
- Lymphoma
Diagnosis: Sinonasal undifferentiated carcinoma (SNUC)
Key points
Aggressive
Rapidly growing
No histologic differentiating features
Presentation: usually older patients
Imaging appearance
Difficult to distinguish from ENB, SCCA, sinonasal adenocarcinoma
Differential diagnosis:
- Esthesioneuroblastoma (ENB)
- Squamous cell carcinoma (SCCA) of the nose
- Meningioma (specifically extracranial nasal meningioma)
- Sinonasal melanoma
- Lymphoma (specifically Non-Hodgkin lymphoma of the nose)
Esthesioneuroblastoma (ENB)
Neuroendocrine malignancy of neural crest origin
Arises from olfactory epithelial of superior nasal cavity
Presentation: adolescent or middle-aged patient with nasal obstruction with epistaxis
May bleed profusely on biopsy
Imaging appearance:
- Dumbbell mass
- Upper portion in intracranial fossa
- Lower portion in upper nasal cavity
- Waist at cribriform plate (blood-brain barrier)
- Calcifications within mass
- Cyst formation at tumor-brain interface
- Destruction of cribriform plate
- Homogenously enhance (CT or MR)
- When large, may have non-enhancing areas of necrosis
Treatment: Resection with radiotherapy
20% have nodal metastasis at presentation
Tendency to recur late
Squamous cell carcinoma (SCCA) of the nose
Malignant epithelial tumor growing from sinus surface epithelium
More common on maxillary antrum than nasal vault—only 30% primarily in nose
Presentation: Older male with sinusitis refractory to medical therapy
Exposures that increase risk:
- Nickel
- Thorotrast
- Possibly formaldehyde and asbestos
Imaging appearance:
- Typically aggressive antral mass
- Invasion and destruction of sinus walls
- Irregular margins
- Indistinguishable from esthesioneuroblastoma if begins high in nasal vault
- Enhancement: heterogenous, less than ENB, adenocarcinoma, melanoma
Treatment: Resection and XRT
With recurrence, 90% < 1 year survival
Meningioma (specifically extracranial nasal meningioma)
Presentation: Middle-aged, typically asymptomatic
Imaging appearance:
- Dural-based avidly enhancing mass
- Hyperostosis in adjacent skull base
- Peritumoral vasogenic edema
- Not associated with cyst formation at tumor-brain interface
Treatment is typically serial imaging, then resection, rarely XRT
Sinonasal melanoma
Neural crest cell malignancy arising from melanocytes in sinonasal mucosa
Presentation: Older patients
Imaging appearance:
- High T1 signal nasal cavity mass
- Can initially resemble nasal polyp on CT
Metastasis: lung, kidney, and breast
Lymphoma (specifically Non-Hodgkin lymphoma of the nose)
Malignant lymphoproliferative disorder arising from variety of immune cell types
B-cell type is most frequent in the paranasal sinuses and is less aggressive
Presentation:
- Nasal obstruction with sinusitis symptoms
- Constitutional symptoms—fever, fatigue, and weight loss
- Unilateral facial swelling
Imaging appearance:
- Dense on non-enhanced CT
- Less enhancement than ENB
- Rarely breaches skull base
Treatment:
- Primarily local XRT
- Chemotherapy if higher stage