Findings
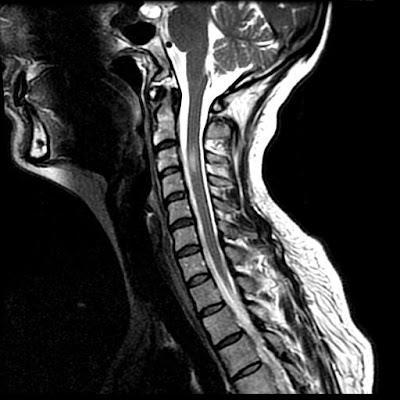
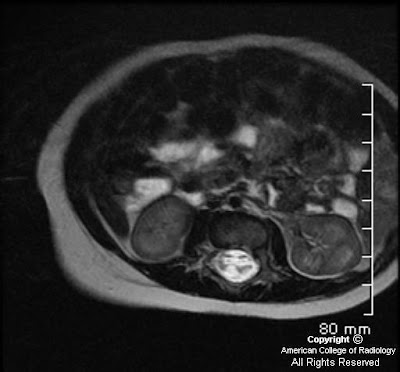
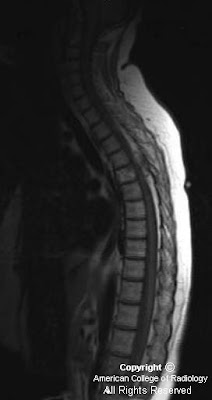
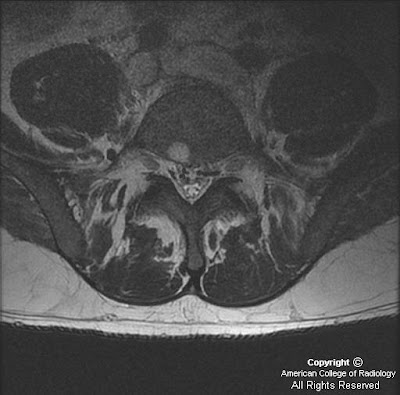
There is a left central disc extrusion at L5-S1 that causes mild to moderate left lateral recess narrowing and nerve root displacement without nerve root compression. At this level there is also contrast enhancement traversing the left laminectomy defect and encasing the disc extrusion, consistent with a wrapped disc. There is enhancement in the left lateral recess, suggesting post-operative fibrosis.
Differential diagnosis:
- Wrapped disc
- Peridural fibrosis
- Epidural abscess
- Epidural metastasis
- Nerve sheath tumor
- Disc pseudobulge
- Intervertebral disc protrusion
- Intervertebral disc extrusion
- Recurrent intervertebral disc herniation
Diagnosis: Lumbar disc extrusion with a wrapped disc
Key points: "Wrapped" disc
Disc herniation (protrusion, extrusion, or fragment) may be caused by trauma, repetitive or acute, and are a common source of pain and subsequent back surgery in the general population. In the acute phase, the herniated disc stimulates a fibrovascular response. A "wrapped disc" is the focal herniation (protrusion, extrusion, or fragment) that is encased in vascular scar tissue stimulated by this response and is evident by enhancement on contrast-enhanced T1-weighted images.
Asymptomatic or low back pain and/or radiculopathy are most common in the lumbar spine at L4-L5 and L5-S1. A wrapped disc is a post-surgical sequela, particularly following surgery for spinal stenosis in which the surgical procedure is more extensive, involving a laminectomy and a medial facetectomy.
Best imaging modality: MR (sequences: sagittal and axial T2WI and T1WI, as well as contrast-enhanced axial and sagittal T1WI)
Other imaging modalities: CT, myelography
Imaging findings
MR: Anterior extradural mass contiguous with the disc space extending into the spinal canal
*Contrast-enhanced T1WI: Peripheral enhancement surrounding the disc herniation or fragment with/without central canal, lateral recess, or foraminal stenosis and cord or nerve root impingement. (*most helpful MR sequence)
Non-enhanced T1WI: Isointense to parent disc
T2WI: Iso- to hyper intense to parent disc
General disc hypointensity and height loss at the level of the herniation, as well as postoperative changes (laminectomy defects, etc), degenerative facet disease, and osteophytes, are common associated findings.
CT:
Non-contrast CT: An anterior extradural soft tissue mass that may displace the nerve root / indent the thecal sac
Contrast-enhanced CT: Mild peripheral enhancement of the disc herniation/fragment
Myelography: An extradural mass that indents the thecal sac and nerve root sleeves
Imaging findings of other common differential diagnoses
Peridural fibrosis: Scar within epidural space after lumbar surgery that infiltrates epidural fat, causing homogeneous enhancement that diffusely surrounds the thecal sac and nerve root; increased in T2 signal relative to adjacent disc herniation
Epidural abscess: A distinct fluid collection in the epidural space with peripheral enhancement on post-contrast images, often associated with findings of diskitis
Epidural metastasis: Elongated (cranial-caudal orientation) enhancing mass with osseous involvement and may demonstrate paravertebral extension
Nerve sheath tumor: Avid enhancement surrounding the nerve root, some of which are in a "dumbbell" shape
Disc pseudobulge: Smooth generalized extension of the disc margin without a focal defect due to "uncovering" of disc related to spondylolisthesis
Intervertebral disc protrusion: Anterior extradural mass contiguous with disc space and triangular in shape with broader base than apex; no enhancement
Intervertebral disc extrusion: Anterior extradural mass contiguous with disc space by a "neck," in which this herniated disc material then widens in the epidural space
Recurrent intervertebral disc herniation: Extradural mass contiguous with intervertebral disc margin, demonstrating enhancement peripherally but without central enhancement
Treatment
Conservative: Anti-inflammatory and pain medications, avoid trauma
Surgical: Repeat surgery to remove herniated disc (protrusion, extrusion, fragment)