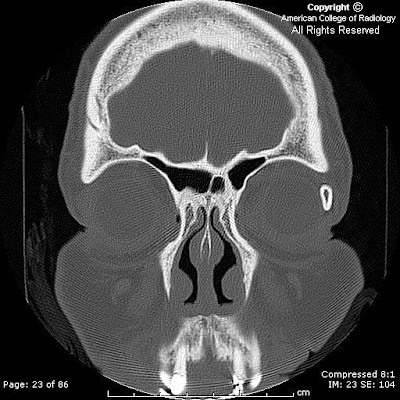
Findings
FindingsA mixed signal (mildly hyper intense on T1-weighted and mixed hyper/hypo intense on T2 weighted sequences) lobular mass with thin rim-enhancement arises from the sella, extending superiorly above the diaphragm sellae (“snowman sign”), deviating the optic chiasm upwards. There is no diffusion restriction, and no susceptibility artifact (on B0 DWI sequences) to suggest hemorrhage. The clivus, and bony sella are intact. A thick rim of dural enhancement dorsal to the clivus is noted.
Diagnosis: Non-hemorrhagic pituitary apoplexy (with underlying macroadenoma)Key points Pituitary apoplexy (PA) (e.g. "necrosis") refers to hemorrhage or infarction of the pituitary.
Commonly there is a pre-existing macroadenoma (65-90%).
Acute clinical syndrome includes HA, visual defects/ophthalmoplegia, AMS, and variable endocrine deficiencies.
Acute imaging features show enlarged gland (T1/T2 isointense) with peripheral enhancement, with or without hemorrhage.
Subacutely – gland T1/T2 hyper intense, and chronically – appears as "empty sella" (filled with CSF) with T1 hypo / T2 hyper signal
T2*GRE sequence is very sensitive in looking for hemorrhagic products.
Sometimes there is subarachnoid hemorrhage.
Thickening/enhancement of adjacent dura (50%) and sphenoid mucosa (80%)
Differential diagnosis includes: Pituitary macroadenoma (non-hemorrhagic), craniopharyngioma, Rathke's cleft cyst, pituitary abscess, primary intrapituitary hemorrhage, or giant thrombosed intrasellar aneurysm.
Patients often suffer from long-term pituitary hormonal insufficiency.
Treatment of PA includes: Steroids, fluid/electrolyte replacement, and sometimes surgical decompression.
Discussion of diseasePituitary adenomas are benign, slow-growing tumors which arise from the adenohypophysis, classified as either micro (< 10mm, 40 %) or macro (> 10 mm, 60%). However, occasionally leptomeningeal metastases can be seen. Patients present with indolent onset of headache, bitemporal hemianopsia (from optic chiasm compression), endocrinologic symptoms.
Pituitary apoplexy (PA), or pituitary necrosis, is usually caused by hemorrhage or infarction of the gland. There can, however, be bland necrosis (without either). The clinical syndrome is of somewhat acute onset with headache, visual defects/ophthalmoplegia, altered mental status, and variable endocrine deficiencies. Pre-existing macroadenoma is very common (65-90%). There is often associated subarachnoid hemorrhage. Thickening / enhancement of the adjacent dura and sphenoid sinus mucosa is very common. Sheehan’s syndrome is a rare peri-partum or post-partum (can be up to 15-20 yrs after index pregnancy) infarction of the pituitary with loss of anterior pituitary hormonal function. Long-term pituitary insufficiency is very common after PA. Treatment may include steroids, fluid/electrolyte replacement, or even surgical decompression.
Radiologic overviewCT (macroadenoma) – isodense to grey matter; fill and expand the sella turcica. CT is excellent for evaluating for sphenoid sinus invasion / bony destruction. Hemorrhage 10%, rarely calcify; pituitary apoplexy appears as hyper dense (acute), or as an "empty sella" if chronic, and very characteristic rim-enhancement.
MR – sellar mass without separate identifiable gland (mass is the gland) with "figure of 8" or "snowman" configuration (coronal), from constriction is caused by diaphragma sellae. Pituitary apoplexy has similar findings, with signal changes based presence/age of hemorrhage. Chronic PA will appear as an "empty sella" (T1 hypo, T2 hyper = filled with CSF). There is thickening / enhancement of adjacent dura in 50% of cases (seen in the presented case), and thickening / enhancement of sphenoid sinus mucosa 80% of the time.