Findings
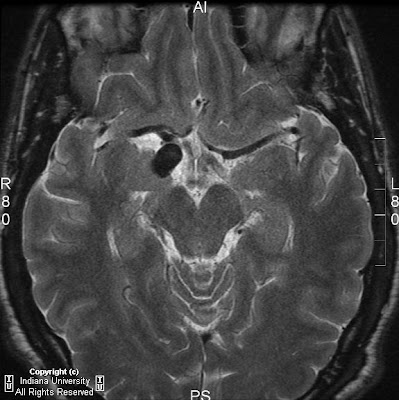
Figure 1: Axial T2 image shows micro-ophthalmia with an irregular-appearing left globe, suggesting a colobomatous deformity.
Figure 2: Axial FLAIR shows absence of the septum pellucidum.
Figure 3: Coronal T1 postcontrast shows absence of the septum pellucidum, a “point-down” appearance to the inferior aspect of the frontal horns of the lateral ventricles.
Figure 4: Axial SPGR: Bilateral atretic optic nerves are seen.
Figure 5: Sagittal T1 FLAIR sequence shows atretic optic chiasm.
Diagnosis: Septo-optic dysplasia/de Morsier's syndrome
Septo-optic dysplasia is characterized by malformations of the optic nerves and tracts, pituitary deficiency, and absence of the septum pellucidum. A small pituitary gland is often identified with an ectopic posterior lobe. Embryologically, the disease is a disorder of midline prosencephalic development. There is secondary degeneration of optic nerve fibers due to cerebral lesions.
The epidemiology of the disease is 1:50,000, with the affected male:female ratio equal to 1. The genetic pattern of inheritance may be either autosomal dominant or recessive.
The most common signs and symptoms in newborns include: hypoglycemia, seizures, apnea, cyanosis, hypotonia, and prolonged conjugated jaundice. Multiple pituitary hormone deficiencies can lead to various presentations. For example, short stature may result from deficiency of growth hormone. Other corresponding endocrinopathies are seen, depending on which hormone is absent. Symptoms may also include color blindness, visual loss, nystagmus, strabismus, mental retardation, spasticity, microcephaly, and anosmia.
Septo-optic dysplasia is frequently associated with other cerebral anomalies. The most commonly associated entity is schizencephaly. Others include perisylvian cortical dysplasia, midline malformation (callosal dysgenesis), ocular malformation (coloboma, anopthalmia, micropthalmia), olfactory tract/bulb hypoplasia and hypoplasia, of the pituitary and olfactory lobes.
CT findings of septo-optic dysplasia include absent septum pellucidum, large lateral ventricles, and small bony optic foramina. MRI is the best imaging tool for diagnosis. Imaging in three planes is crucial to identify all findings. Coronal MRI images demonstrate absent septum pellucidum, flat roof of frontal horns, and a “point down” appearance of the inferior aspect of frontal horn. A small optic chiasm/nerve, thin pituitary stalk, and posterior pituitary ectopia can also be seen when fat saturation sequences are used. T1 postcontrast imaging demonstrates enhancement of infundibulum and an ectopic posterior pituitary lobe.
Differential diagnosis includes syndromes overlapping with septo-optic dysplasia, such as optic infundibulum dysplasia (OID) with schizencephaly but normal septum pallucidum, Kallmann's syndrome, and lobar holoprosencephaly.
Untreated septo-optic dysplasia can lead to hypothalamic and pituitary crisis or sudden death from hypocortisolism. Treatment of septo-optic dysplasia consists of hormonal replacement therapy.