Findings
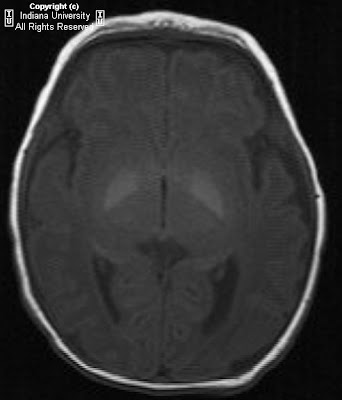
Figure 1: On the right, the mastoid air cells are under pneumatized. There is no identifiable external auditory canal.
Figure 2: A thick bony plate is visualized in the expected area of the external auditory meatus.
Figure 3: The facial nerve is identified.
Figure 4: There is a small amount of bone in the attic of the middle ear cavity but no formed malleus or incus is identified. This suggests rudimentary and/or hypoplastic ossicles. However, a normal morphology and location of the stapes is seen. The internal auditory canal is normal in caliber.
Figure 5: The apical and basal turns of the cochlea are within normal limits.
Diagnosis: Microtia
Microtia, which is a congenital deformity of the pinna and occurs more commonly in boys, is seen 1 in 8,000-10,000 births. It can be unilateral or bilateral. The etiology is unknown, but related to a variety of genetic factors. It is associated with other congenital syndromes, such as Townes-Brocks syndrome, Nager syndrome, and Miller syndrome. Some clinicians consider microtia to be a manifestation of the oculo-auriculo-vertebral spectrum (OAVS), where there are also facial, vertebral, and renal abnormalities. Renal abnormalities usually warrant a renal ultrasound, looking for abnormalities such as renal agenesis, hypoplasia, and crossed ectopia.
Microtia can be graded as follows:
- Grade I: A slightly small ear with identifiable structures and a small but present external ear canal
- Grade II: A partial or hemi-ear with a closed off or stenotic external ear canal producing a conductive hearing loss
- Grade III: Absence of the external ear with a small peanut vestige structure and an absence of the external ear canal and ear drum
- Grade IV: Absence of the total ear or anotia
Imaging of microtia is largely performed to form a template for further management options. Initially, a CT scan of the head (and/or temporal bones) is used to evaluate the exact middle and inner ear anatomy. Further staging by CT is recommended in order to avoid lesions to the facial nerve which most often can be displaced from its original location in patients with microtia, and also to assess for prognosis. In those patients being evaluated for surgical intervention, the following structures should be evaluated for planning surgery: the external auditory canal, bones (temporal, zygomatic, and the mandibular condyle), vessels (carotid canal, sigmoid sinus, and the jugular bulb), tensor tympani muscle and grade of mastoid pneumatization, ossicles, cochlear turns, vestibule, semicircular canals, facial nerve canal, and finally, the internal auditory canal. These pre-operative images substantially reduce the risk of facial nerve palsy, bleeding and worsening of hearing.
On axial CT images of the head, one can evaluate for either stenosis or complete atresia of the external auditory canal. At the same time, bone erosion by a secondary cholesteatoma or epidermoid cyst can be identified. One study showed the tympanic and/or mastoid portions of the temporal bone as commonly hypoplastic on CT images of patients with microtia. Dysplasia of the mandibular condyle and the zygomatic arch were also shown. Changes of the ossicles are frequently present and include dysplastic shape, diminution, thickening, axis rotation, or complete absence. Occasionally, the lateral semicircular canal is hypoplastic in patients with severe middle ear involvement. The internal auditory canal is rarely dysplastic in patients with microtia.
Microtia repair is complex. Testing is done initially to determine whether hearing is normal. If hearing is normal and a canal is not visible externally, a CT will be done to determine whether a rudimentary canal exists. The earliest age surgery can be attempted is three years, but will vary according to the graft material used. In some cases, patients may have to wait as long as 6 years of age. Exploration involves cautiously avoiding the facial nerve while drilling a canal through solid bone. A cartilage framework, usually derived from costal cartilage, is created and anchored beneath the skin of the mastoid area. Once it is well attached to the surface skin, a post-auricular crease is created in a second operation.