Findings
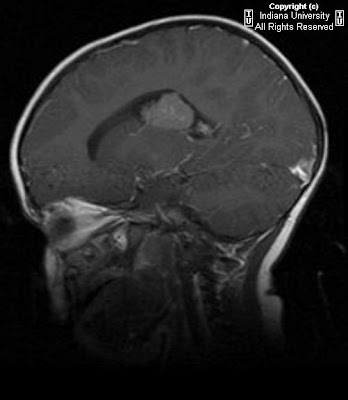
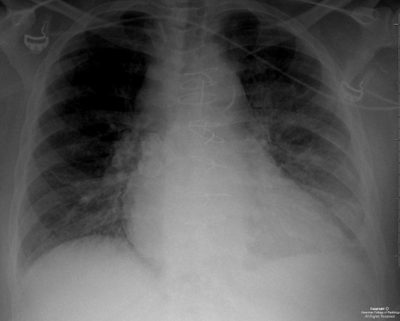
Axial (Figure 1) and coronal (Figure 2) non contrast CT images, as well as an axial T2 MRI image (Figure 3), demonstrate symmetrically enlarged lacrimal glands which protrude anterior to the lacrimal fossa bony contours. Homogeneous enhancement is identified on the selected contrast enhanced axial CT image (Figure 4). There is no evidence of adjacent bony orbit invasion. An AP chest radiograph (Figure 6) demonstrates predominantly right hilar lymphadenopathy with “eggshell” calcification and bilateral reticulonodular opacities within the lung parenchyma.
Diagnosis: Sarcoidosis involving the lacrimal glands
20 to 25 percent of patients with systemic sarcoidosis develop ophthalmic manifestations usually between the third and fifth decades. The most common finding is inflammation of the uveal tract. Less commonly, patients will present with inflammation of the optic nerve or orbital involvement including pseudotumor like intraorbital masses, extraocular muscle enlargement, or lacrimal gland infiltration and hypertrophy. Isolated orbital disease is uncommon and usually limited to the lacrimal glands.
Lacrimal gland involvement occurs in approximately 15-28% of patients; usually as painless bilateral gland swelling evident on physical examination. In many cases, lacrimal gland involvement may occur long before lung and other organs are affected, thereby aiding in the early diagnosis of systemic sarcoidosis.
Imaging studies such as orbital CT and MRI are an integral part of early diagnosis. CT findings include symmetric enlargement of the lacrimal glands with diffuse homogeneous post contrast enhancement. There may be associated medial displacement of the optic globes as well as proptosis. Pertinent negative findings include the absence of adjacent bony orbital invasion and destruction. MRI is optimal to evaluate for additional orbital involvement such as optic nerve infiltration which manifests as a thickened and enhancing intraorbital nerve. An MRI of the brain is also recommended to evaluate for extension into the intracranial optic pathways and to exclude findings of coexisting neurosarcoidosis.
While diagnostic imaging tests may reveal findings highly suggestive of lacrimal gland sarcoidosis; definitive diagnosis requires biopsy of the glandular tissue and histopathologic assessment. Noncaseating granulomas characterized by clustered epithelial cells, central giant cells and abundant surrounding lymphocytes are characteristic.
The mainstay of therapy is high dose systemic steroids, usually oral prednisolone for approximately two weeks, followed by gradual tapering after the inflammation appears controlled. Some patients may require maintenance doses for several weeks to months.