

Findings
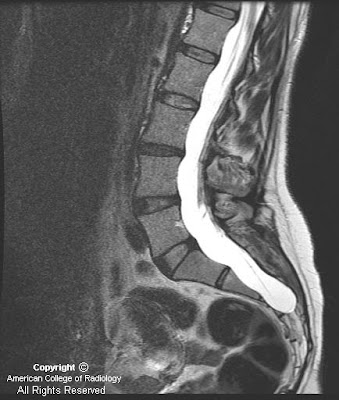
Figure 1 and Figure 2: Axial and sagittal CT images demonstrate dural ectasia with a capacious thecal sac to the level of the sacrum.
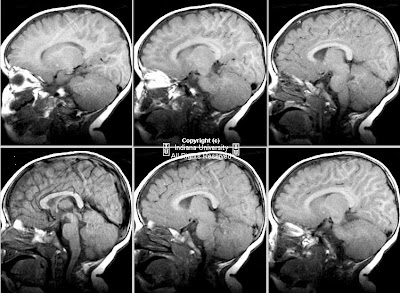
Figure 3 and Figure 4: Sagittal T1 postcontrast and T2-weighted MR images reveal a dilated terminal thecal sac without a tethered cord. Benign subtle scalloping of the posterior margin of the lumbar and sacral vertebral bodies (Figure 4) is best visualized on the T2-weighted images.
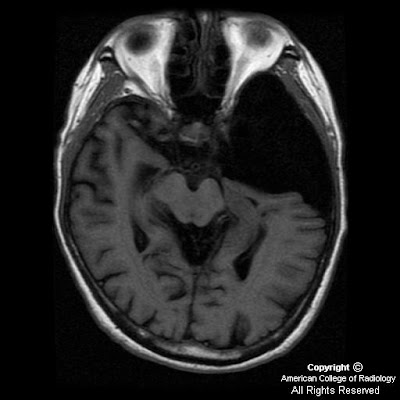
Figure 5 and Figure 6: Axial T1 postgadolinium and axial T2-weighted MR images reveal a dilated thecal sac with root sleeve prominence.
Differential diagnosis:
- Idiopathic dural dysplasia
- Neurofibromatosis I
- Marfan syndrome
- Ehlers-Danlos syndrome
- Homocystinuria
- Achondroplasia
- Hurler syndrome (MPS IH)
- Syringomyelia
- Ankylosing spondylitis
Diagnosis: Idiopathic dural dysplasia
This case is an example of an expanded dural sac with posterior vertebral scalloping of the lumbar spine and sacrum. Findings include a capacious thecal sac with smooth scalloping of the posterior aspect of the involved vertebral bodies. Dural dysplasia most often occurs in the lumbar spine but can involve the cervical and thoracic spinal canal as well. The case described herein is mild as there is no resulting kyphoscoliosis or erosion of the pedicles which occurs in more severe cases. Making the diagnosis of dural dysplasia requires that one excludes other causes of the expansion of the canal such as syrinx, tumor or meningeal cyst. The differential diagnosis and etiology of dural dysplasia is extensive and should be distinguished from meningeal cysts. The patient in this case had no known cause for the dural dysplasia and only complained of back pain.
Meningeal cysts
- Type I meningeal cysts: are extradural cysts that do not contain nerve root fibers. Type IA cysts are extradural arachnoid cysts. Occult sacral meningoceles (OIM) are considered type IB meningeal cysts which are also extradural and do not contain nerve root fibers. OIMs present with smooth remodeling and enlargement of the sacral canal with an extradural arachnoid sacral cyst adjacent to the thecal sac.
- Type II meningeal cysts are extradural cysts that contain nerve root fibers. These include Tarlov cysts and spinal nerve root diverticula. Tarlov cysts are cystic dilatation of the sacral root pouches with associated bone erosion which may or may not be symptomatic.
- Type III meningeal cysts are true intradural arachnoid cysts.