Findings
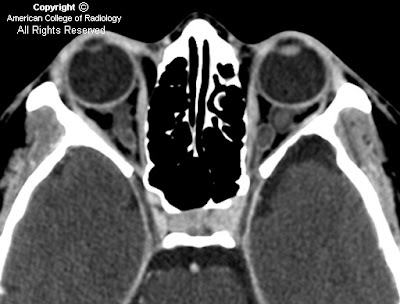
FindingsNon-contrast series demonstrates multiple areas of low attenuation in the subarachnoid space (Figure 1 and Figure 2).
The second CT series demonstrates a heterogeneous, predominantly fatty lesion in the left aspect of the middle cranial fossa extending toward the orbital apex extending across the tentorial notch (3). Focus of fat is seen extending into the region of the internal audiory canal (IAC) (Figure 3).
Sagittal T1 image demonstrates multiple punctuate areas of high signal, consistent with fat droplets (Figure 4). Axial T1 post gadolinium image demonstrates a lesion with fatty and cystic components extending into the left internal auditory canal, (Figure 5). Axial T1 post gad fat sat images demonstrate the extensive fatty component, which saturates on this sequence, (Figure 6).
Diagnosis: Ruptured intracranial dermoidIntracranial dermoids are rare, accounting for less than 1% of brain “tumors” in the US. They are more common in men than women. Unlike teratomas, they are not true neoplasms. Histologically, dermoid cysts are inclusion cysts lined by epithelium. They are caused by displaced ectodermal elements during development around the time when the neural tube closes at midline. This explains their usual near-midline location. Dermoid and epidermoid cysts are often discussed together due to their similar appearance and origin. In contrast to epidermoids, dermoids have an epithelial lining further differentiated into hair, sebaceous glands, and sweat glands. The two can be differentiated radiographically based on their typical locations and imaging characteristics. Click here for a quick comparison of the two.
Dermoid cysts are benign and slow growing, and are usually located near midline within the posterior cranial fossa, parasellar, and sub frontal areas. Symptoms depend on the size, location, and mass effect on adjacent structures. Patients may present with visual disturbances, seizures, diabetes insipidus, or headache. Intraventricular dermoids are most commonly in the fourth ventricle and rarely cause hydrocephalus. Spontaneous rupture, as in this case, can incite a chemical meningitis, resulting in recurrent headaches or seizures. Although rare, the resultant meningeal inflammation can cause vasospasm, and even stroke and death. Traumatic rupture has also been reported. In addition to intracranial involvement, dermoid cysts may also be seen in the scalp, skull, orbit, spine, nasal/oral cavity, and neck. Ovarian (abdominal) dermoids are actually well-differentiated and organized teratomas.
On CT, dermoid cysts are low attenuation, well-circumscribed, and cystic. Wall calcifications may be seen (about 20% calcify) and enhancement is rare. Fat in the subarachnoid and ventricular spaces suggests rupture, and occasionally fat/fluid levels may be seen. The resorption of subarachnoid fat is variable, and may be present up to 6 years following surgery.
On MR, dermoids typically have signal characteristics of fat – hyperintense on T1 weighted images, and hypointense on T2. Fat suppression sequences may be helpful. On both CT and MR, calcification, follicles, and debris can give dermoids a heterogeneous appearance. Dermoids are never associated with vasogenic edema, and as mentioned above, rarely cause hydrocephalus.
Treatment usually consists of complete surgical resection, depending on the size, location, and effects on local nerves and vessels. There is a risk of causing chemical meningitis during removal of the dermoid cyst if its contents spill into the subarachnoid or ventricular spaces. Recurrence is rare.