Findings
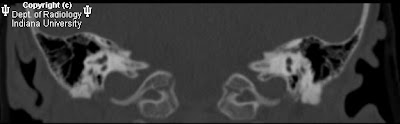
FindingsCT head: Multiple areas of intracranial hemorrhage in the left hemisphere. There is thrombosis of the superior sagittal sinus and a left cortical vein. Two focal hyper dense masses are seen in the left occipital lobe and the medial left temporal lobe.
MRI and MRV brain: Multiple intraaxial masses in the left cerebral hemisphere seen in the temporal lobe, basal ganglia, occipital lobe, and genu of the corpus callosum which show blooming artifact on gradient echo images, and a hypo intense rim on T2-weighted images. There is a large left hemispheric venous angioma with dilated medullary veins which drain to the internal cerebral vein best seen on post contrast images. The anterior superior sagittal sinus is hypoplastic or stenotic.
Cerebral angiogram: No evidence of aneurysm or AVM. There is a large left venous angioma draining the entire left hemisphere into the internal cerebral vein then to the vein of Galen. Left cortical vein thrombosis, nonocclusive posterior superior sagittal sinus thrombus, and absence of the anterior superior sagittal sinus.
Differential diagnosis:
- Multiple arteriovenous malformations
- Multiple cavernous malformations with large venous angioma and sagittal sinus stenosis and partial thrombosis
- Multiple cavernous malformations with venous stasis due to sinus stenosis and partial thrombosis
- Multiple hemorrhagic metastases with leptomeningeal enhancement
- Multiple calcified metastases and leptomeningeal enhancement
- Sturge Weber with arteriovenous malformations
Diagnosis: Multiple cavernous malformations (AKA cavernomas) with associated large hemispheric developmental venous anomaly (AKA DVA or venous angioma)Key pointsThe diagnosis is favored over cavernomas with venous stasis because:
- There is a known association between cavernous malformations and developmental venous anomalies.
- The cerebral angiogram shows an embryologic drainage pattern.
Cavernous malformationsClinical
- Presentation: Seizure 50%, neurologic deficit 25%
- Pathology: Collection of endothelial lined, blood filled vessels without intervening normal brain
- Genetics: Multiple cavernous malformations can be seen with an autosomal dominant chromosomal abnormality
- Prevalence: 0.5%
- 75% solitary, sporadic lesion
- 10-30% multiple, familial
- Hemorrhage rate: Sporadic 0.25-0.75%
- Associated anomalies:
Developmental venous anomaly
Superficial siderosis
Cutaneous findings: café au lait spots, cherry angiomas
Imaging:
- Cerebral angiography: Most common angiographically occult vascular malformation, i.e. not detectable. May be associated with a venous angioma
- MRI: Popcorn ball appearance; mixed signal intensity core with a hypo intense hemosiderin rim; prominent susceptibility artifact ("blooming"). Diffusion usually normal
- CT: Negative in 30-50%; may appear as an ovoid hyper dense lesion. 40-60% have Ca2+
Developmental venous anomaly (AKA venous angioma)Clinical
- Embryology: Felt to be secondary to arrested medullary vein development resulting in persistence of large primitive deep embryonic white matter veins
- Most common vascular malformation at autopsy
- Usually asymptomatic
- 15-20% associated with cavernous malformations
- Radially oriented dilated medullary veins
- Hemorrhage risk increases with occlusion of the draining vein. Risk felt to be 0.15% per lesion/year
- Multiple can occur with blue rubber-bleb nevus syndrome
Imaging
- Prevalence: 2.5-9% of MRI scans
- Contain normal intervening brain
- Dilated medullary veins have the "medusa head" or umbrella-like appearance; located often at the angle of the ventricle; stellate, tubular vessels converse on a collector vein which drains into a dural sinus/ependymal vein
- Differential diagnosis includes dural sinus occlusion with venous stasis and collateral drainage.
- Cerebral angiography: Most common angiographically occult vascular malformation, i.e. not detectable; may be associated with a venous anomaly
- MRI:
T1 and T2 weighted images: Can be normal if small, or see flow void if large enough
T1+C: Stellate pattern of tubular vessels of strong enhancement draining to a collector vein to a dural sinus/ependymal vein
MRV: Demonstrates medusa head and drainage pattern
CT: Usually normal. Parenchymal hemorrhage if draining vein occluded