Findings
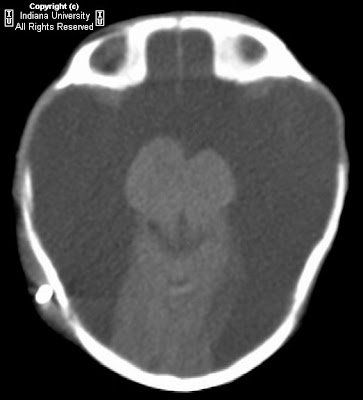
Figure 1 and Figure 2: Select axial non-contrast CT images demonstrate dense symmetric calcification in the basal ganglia (Figure 1) and dentate nuclei (Figure 2). Cortical atrophy is also seen.
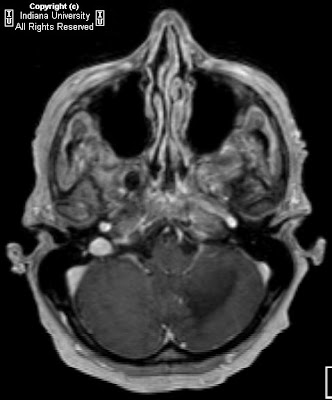
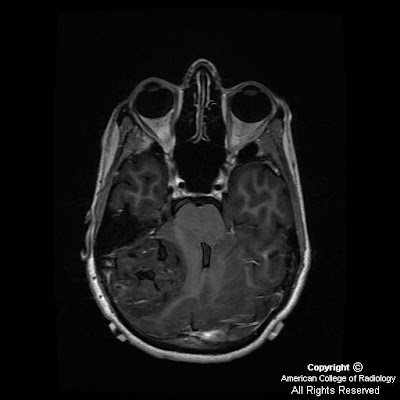
Figure 3: Axial T1-weighted image demonstrates symmetric cerebellar hyperintense foci corresponding to calcification seen on CT.
Diagnosis: Fahr disease (Idiopathic familial basal ganglia calcification)
Fahr disease (Idiopathic familial basal ganglia calcification, bilateral striopallidodentate calcification, Familial cerebrovascular ferrocalcinosis) is a rare disorder characterized by idiopathic basal ganglia calcification with associated cognitive and neurobehavioral manifestations.
Calcification is found primarily in the globus pallidus, but the putamen, caudate, thalamus, cerebellum (especially dentate nucleus), corona radiata, and subcortical white matter can also be affected. There are no detectable abnormalities of calcium or phosphate metabolism. Patients develop progressive parkinsonism, dystonia, and neuropsychiatric disturbance.
There is a bimodal pattern of onset. Those affected in early adulthood may be asymptomatic in the first two decades, despite the presence of basal ganglia calcification. The disease presents with schizophreniform psychosis. A second peak of onset is seen in late middle age, when patients present with subcortical dementia or Parkinsonian symptoms, which are permanent and progressive. Paroxysmal dystonic choreoathetosis and seizures are common. Eventually patients develop symmetrical spastic paralysis progressing to a decerebrate state.
The disease process involves the deposition of calcium in the walls of the capillaries and larger arteries and veins. Other elements, including magnesium, zinc, aluminum, and iron have also been found deposited in the vessels. No definitive treatment is available.
In studying a three-generation family with an autosomal dominant form of the disease, Geschwind et al, established that the chromosomal locus, IBGC1, lies on chromosome 14, and found that this form of the disease demonstrates genetic anticipation. Autosomal recessive inheritance has also been documented. The disease demonstrates variable expressivity.
CT images demonstrate bilateral, symmetric calcification in the globus pallidus, cerebellum, and white matter. On T1-weighted MR images calcifications are hyperintense, while on T2-weighted and FLAIR images, calcification may be hypo- or hyperintense. T2 hyperintense regions in the white matter, which do not correspond to calcification can also be seen. This finding may reflect progressive inflammation.
Differential diagnosis for inherited and acquired basal ganglia calcification
Postinflammatory causes:
- Tuberculosis
- Toxoplasmosis
- Cystercercosis
- Congenital HIV
Endocrine causes:
- Hyperparathyroidism
- Hypoparathyroidism
- Pseudohypoparathyroidism
- Hypothyroidism
Congenital causes:
- Tuberous sclerosis
- Down syndrome
- MELAS
- Neurofibromatosis
Toxic causes:
- Exposure to carbon monoxide
- Chemotherapy
- Radiation therapy
- Lead intoxication
In addition, incidental basal ganglia calcification is seen frequently on CT imaging in patients over the age of 50.