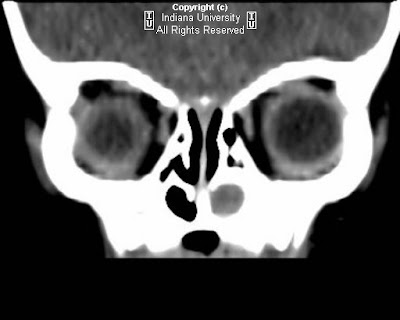
Findings
Small hypodense lesion is noted along the right superolateral orbital rim (Hounsfield units approximately -6), causing smooth contoured external depression of the adjacent bone. No evidence of bone destruction or abnormal periosteal reaction. The globe, extraocular muscles, and orbital soft tissues appear normal.
Differential diagnosis:
- Epidermoid cyst
- Dermoid cyst
- Subperisosteal abscess
- Eosinophilic granuloma
- Metastasis (e.g., neuroblastoma).
Diagnosis: Periorbital epidermoid cyst
Key points
Dermoid and epidermoid cysts are found in a variety of locations around the skull and midface, typically classified as true choristomas. Choristomas are tumors that are composed of tissues not normally found at their site of occurrence, instead arising from the sequestration of surface ectoderm by closure of the underlying suture lines, neural tube closure, and diverticulation of the cerebral hemispheres. Dermoid cysts contain ectoderm and skin elements, whereas epidermoid cysts contain ectoderm but no skin elements. Histologic examination is necessary to differentiate the dermoid cyst with its squamous epithelium lining containing dermal appendages from the epidermoid cyst, which has no dermal appendages. Dermoid and epidermoid cysts are most commonly seen in midline and frontotemporal locations, followed by parietal locations. Midline locations include the anterior fontanel, glabella, nasion, vertex, and subocciput. Nasal dermal sinuses and dermoid and epidermoid cysts occur at multiple locations and may be associated with external skin ostia or deep sinus tracts, which may potentially extend intracranially.
Complete surgical excision of the dermoid or epidermoid is the recommended management, as the contents of the cystic lesion are irritating and may result in lipogranulomatous inflammation if ruptured. Deep orbital cysts extending into the orbital roof, temporal fossa, or intracranially may require neurosurgical assistance for removal.
Radiology
Careful investigation is important to distinguish deep from superficial lesions. CT attenuation will vary depending on cyst content. Similarly, the signal intensity at MR imaging depends on the contents of the cyst and may range from pure fluid signal intensity in an epidermoid cyst to a more complex signal intensity in a dermoid cyst. Epidermoid cysts also typically have bright signal intensity on isotropic diffusion-weighted MR images. With nasal dermoid cysts, high-resolution surface coil MR imaging is useful in determining if there is a connecting sinus tract in the prenasal space to the foramen cecum.